Hylomorph
Formative evaluation of an implantable pouch to fit pacemakers and neurostimulators.
Hylomorph AG is a clinical-stage medical device company developing innovative materials designed to transform the biocompatibility of implantable medical devices. Founded by Simone Bottan, Aldo Ferrari and Francesco Robotti in 2014, Hylomorph is a Medtech startup company and spin-off of the Swiss Federal Institute of Technology.
The product
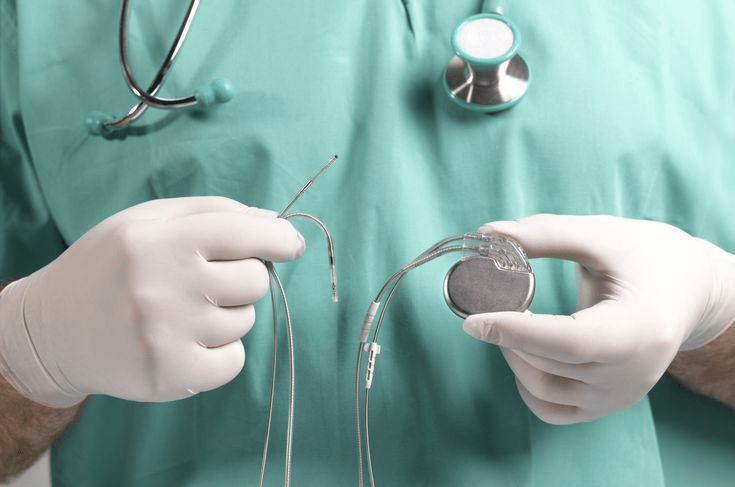
Hylomate Pouch is an implantable, seamless pouch manufactured from surface micro-engineered biosynthesize cellulose.
Hylomate is designed to fit cardiac implantable electronic devices (such as pacemakers) and neurostimulators with the aim of preventing post-operative complications.
What our client wanted
Since 2017, Hylomorph has been one of Bergo’s most valued clients. We have assisted with several past studies (both formative and summative) involving two of their products: Hylomate Sheet and Hylomate Pouch.
At the beginning of 2022, Hylomorph approached Bergo to assist them with conducting a formative evaluation of Hylomate Pouch. A key objective of the study was to evaluate the handling of the product. For instance, can an implantable device, such as a pacemaker or neurostimulator, be easily inserted into the pouch?
What Bergo delivered
Initial discussions focused on deciding where to conduct the evaluation. In previous studies, venues in London and Birmingham were used. However, given how highly specialised the intended users are and to avoid reusing participants, a decision was taken to conduct the study at two different locations:
- A clinician simulation suite in Stockport (near Manchester) – premises owned by our recruitment partner Acumen Fieldwork
- A clinical and surgical skills training facility in Clydebank (near Glasgow) – located in the Golden Jubilee Research Institute
In both venues, an operating room was recreated, consisting of a hospital bed, medical mannequin, surgical table and various tools and equipment. Pig’s belly tissue was also positioned on the mannequin’s chest so that participants could simulate the implantation procedure.
A total of nine participants took part, consisting of cardiologists, cardiac electrophysiologists, neurosurgeons, and cardiac surgeons. In each video-recorded, one-to-one session, the following steps were carried out:
- Training: the participant received a short training session to reflect the anticipated training in practice (carried out by a Hylomorph colleague)
- Distraction task: asking the participant to perform a distraction task to evaluate possible training decay
- Using product: observing the participant simulate the use of the product, consisting of opening packaging, removing sterile contents, inserting the implantable device and coiled-up lead into pouch, suturing the pouch close and implanting device
- Knowledge task questions: asking the participant questions on their understanding of information for safety
- Interview: questioning the participant to establish the root cause of any use errors or incorrect responses to knowledge task data questions.

